Medical Clowns – No Laughing Matter
Israeli researchers find that medical clowns contribute significantly to the achievement of medical therapeutic goals.
You see them stroll around in the hospitals’ toughest wards with their red noses, colorful clothes, and unwavering smiles, spreading laughter and cheerfulness wherever they go. They are the medical clowns: trained professionals whose goal is to change the hospital environment through humor.
A new study tested and categorized the skills of medical clowns and found that their importance goes far beyond contributing to a patient’s good mood. The researchers identified 40 different skills of medical clowns, including establishing an emotional connection and creating a personal relationship with the patient, expressing the patient’s frustrations and difficulties to the medical staff, increasing the patient’s motivation to adhere to medical treatment, distracting the patient from pain, and creating a joyful atmosphere.
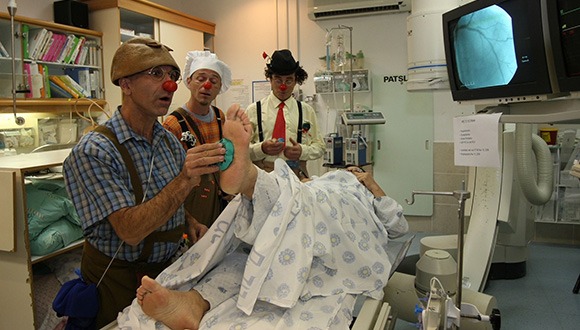
Medical clowns working alongside other therapists (Photo: The Dream Doctors Project, Medical Clowning in Action)
Not Just for Entertainment Purposes
The research was conducted under the leadership of Prof. Orit Karnieli-Miller, with Dr. Lior Rosenthal, both from the Department of Medical Education at TAU’s Sackler Faculty of Medicine, in collaboration with Ms. Orna Divon-Ophir, Dr. Doron Sagi, Prof. Amitai Ziv and Ms. Liat Pessach-Gelblum from the Israel Center for Medical Simulation (MSR). The study was published in Qualitative Health Research, a leading journal in the field of health.
The researchers show that not only do medical clowns help the patients and their family members, but also the medical team and the achievement of treatment goals.
Through use of different communication skills, clowns make it easier for the patient to cooperate with various treatments. The medical clowns work in a team with other therapists, know how to intervene and help whenever an argument or crisis should arise to advance treatment.
“From the moment they enter the room, the clowns form a bond with the patients, strengthen them, and give them power and status within the medical system.” Prof. Orit Karnieli-Miller.
Decoding their “Secret Magic”
Studies conducted throughout the years have shown the clowns’ positive influence on the hospital environment through humor, as well as helping patients deal with pain. However, no studies have empirically mapped the skills they use and their therapeutic goals to help understand their “secret magic.” In addition, there was a lack of broad understanding of how clowns can help children, teenagers, and their parents in various challenging situations of distress and difficulty, as well as how they can help patients and medical teams achieve treatment goals. This lack of appreciation of the potential benefits of utilizing the services of medical clowns meant that patients and medical teams would occasionally be reluctant to cooperate with them.
As part of the new study, the researchers focused on qualitative, in-depth systematic identification of the skills of medical clowns through observation and analysis of their actions in challenging encounters with adolescents, parents, and medical staff.

Medical clowns help patients and medical teams achieve treatment goals (Photo: The Dream Doctors Project, Medical Clowning in Action)
The team analyzed videotaped sessions of medical clowns in various simulated situations and conducted in-depth interviews with expert medical clowns. The researchers identified 40 different skills used by the medical clowns to achieve four therapeutic goals:
1) building a relationship and connecting to the needs and desires of the patients
2) dealing with emotions and difficulties
3) increasing the patient’s motivation to adhere to the treatment plan
4) increasing the patient’s sense of control and providing encouragement to patients
The clowns examined in the study were trained and recruited by the “Dream Doctors Project”, a non-profit association that employs medical clowns as part of the paramedical system in Israeli hospitals, and trains them to work within multi-disciplinary teams. The Tel Aviv University researchers collaborated with the Israel Center for Medical Simulation (MSR), which created a simulation-based workshop focused on developing the skills of experienced medical clowns.
“From the moment they enter the room, the clowns form a bond with the patients, strengthen them, and give them power and status within the medical system,” explains Prof. Karnieli-Miller. “They do this through an initial connection to the patients’ voice, and even to the patients’ reluctance to implement therapeutic recommendations – an emotional connection that often results in the patient changing their position and cooperating with the medical staff.”
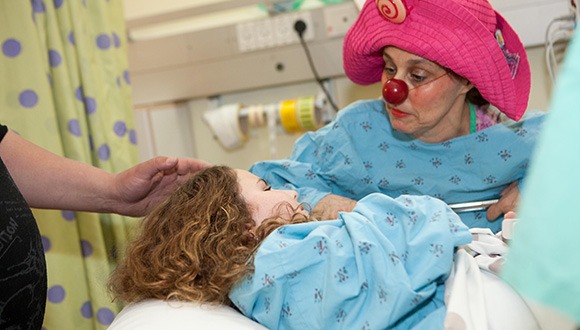
Providing the patient with an increased sense of control and courage to face their challenges (Photo: The Dream Doctors Project, Medical Clowning in Action)
According to Prof. Karnieli-Miller the medical system is hierarchical, and it is not always easy for patients to navigate. Therefore, one of the skills of medical clowns is to place themselves in the lowest position in the medical setting. By doing so, they empower the patients by giving them a sense of power and control, including the choice of whether to allow the clown to enter the room as well as to dictate the nature of the patient’s role vis-à-vis that of the clown. This provides the patient with an increased sense of control and courage to face their challenges.
The researchers emphasize that the clowns are very aware of the emotional difficulty associated with staying in a hospital and dealing with an illness. To help deal with these issues, the clowns sometimes distract the patient by using props, humor, and imagination. Other skills include allowing the patient to direct their frustrations towards them, away from medical staff or parents.
Depending on the situation the clowns may also use a comforting touch, soothing music, empathetic listening, or a reinforcing statement to provide an environment where the patient feels comfortable to express their feelings. A patient’s ability to gain legitimacy is important and is strengthened by the clowns.

Prof. Orit Karnieli-Miller
“Mapping the skills and goals of the medical clowns improves their understanding of their role and may help other health professionals appreciate their work methods and the benefits of incorporating these methods into their own practices when faced with similar challenges” Prof. Orit Karnieli-Miller.
Learning from Medical Clowns’ Methods
“Mapping the skills and goals of the medical clowns improves their understanding of their role and may help other health professionals appreciate their work methods and the benefits of incorporating these methods into their own practices when faced with similar challenges,” adds Prof. Karnieli-Miller.
“This research is important because it allows the clowns to enhance their training program and refine their diverse skills to achieve the various therapeutic goals appropriate for different patients, as well as helping health professionals collaborate with the medical clowns. If professionals in the healthcare field gain a clear understanding of how and when to cooperate with the medical clowns, they will be able to help patients overcome challenges, and at the same time they may be more tolerant of the clowns’ ‘disruption’ of the hospital care regimen. This appreciation of the clowns’ contribution will provide the clowns with the time and space to connect with patients and help and encourage patients to become more active participants in their treatment plan,” she concludes.
Featured image: Medical clown with happy customer (Photo: The Dream Doctors Project, Medical Clowning in Action)